Which of the Following Contains the Largest Number of Atoms
How many grams are in 205 x 1023 molecules of dinitrogen pentoxide. Total atoms 2 atoms For AI₂S0₄₃.

The Number Of Atoms Present In One Mole Of An Element Is Equal To Avogadro Number Which Of The Following Element Contains The Greatest Number Of Atoms
5 g of NH 3 2017 N A atoms.

. 2 atom of Chlorine. Thus 1g of butane has greatest number of atoms. 2 moles of molecule contains atoms.
1mg of Na 1 atom in 1 molecule N x 10 -3 23 434 x 10 -5 x N atoms. 1 mol dinitrogen pentoxide d. 2 mol carbon monoxide e.
One mole of any substance is equal to the value of 6023 x 10. One mole of C₁₀H₈ contains the largest number of atoms option 4 First step. 30 moles of ceH2SO3 c.
Which one of the following contains largest number of atoms -. Which of the following contains the largest number of atoms -. Which of the following compounds contains the largest number of atomsA.
17 g of NH 3 4N A atoms. Determine the number of atoms in each mole of the given elements. And 506 10-3 mol of SO3 contains even more moles than 00553 g of SO3.
Which of the following contains the maximum number of atoms and why. Furthermore since the MM of SO3 is 801 gmol you have 00553g 801 g mol 690 104molSO3. Obviously there are more oxygen atoms in SO3.
The maximum number of atoms is present in 28th December 2020 450 PM. 100 mole of H3PO4. I 1 g of Li s Given.
20 moles of ceH3PO4 b. Thus 1ml of H 2 O has largest number of atoms. Which of the following compounds contains the largest number of atoms.
Molecular mass of Water H 2 O is 18gmol. Chemistry questions and answers. Which of the following contains the largest number of atoms -.
1 mol phosphorus trichloride PCl 3 c. The average mass of an oxygen atom is 53 10-26 kg. The largest number of atoms present in i 1 g of Li s Solution.
So the number of atoms in each case is directly proportional to the number of moles. 025 mole of molecule contains atoms. A mole fraction indicates the number of chemical elements.
So 0142 mole contains number of Li atoms. To calculate the moles we use the equation. Now we have to calculate the number of Li atoms.
Correct option is A a 05g atom mole of Cu05602310 23. Calculate the kinetic energy of a mole of oxygen atoms all moving at a speed of 400 ms 1000 mph In the. 0 2 3 1 0 2 3.
100 mole of NH4. A 4g of H2 b 16g of O2 c 28g of N2 d 18g of H2O. 1 mol carbon dioxide CO 2 e.
1 mole of carbon tetrachloride CCl 4 d. 80 moles of ceHBr My logic would tell me that every mole has 6022times1023 atoms in it. 64 g of SO 2 3 N A of atoms.
Total number of atoms 00092 x 6022 x 10 23 0054 x 10 23 atoms. Which of the following 850 g samples contain the greatest number of atoms. 6022 x 1023 atoms.
Molar mass of Li 7 gmole. Which of the following contains the largest number of atoms. 1mg of N 2 2 atoms in 1 molecule 2N x 10 -3 28 714 x 10 -5 x N atoms.
D 8g of SO 2. 4 g of hydrogen contains the largest number of atoms. First we have to calculate the moles of Li.
How many atoms are in 100 mole of water. A glass jar contains one molecule each of rock salt NaCl washing soda Na2CO3 sodium sulfate Na2SO4 and chalk CaCO3. 1ml of H 2 O 3 atoms in 1 molecule 1g of H 2 O 3N 18 241 x 10 -4 x N atoms.
Total atoms 8 atoms For CI₂. 1 mole of NH 3 4N A Atoms. How many atoms are in 1 mole of calcium.
A 4 g of hydrogen molar mass 2 corresponds to 2 moles of molecules or 4N number of atoms where N is the Avogadros number 6. Which of the following contains the largest number of atoms -. Which of the following samples contains the largest number of atoms.
What is the empirical formula. 40 moles of K2SB. Now 1 mole of molecule contains atoms.
All of these contain the same number of atoms. Outcome 7 DOK 3 A C6H8O7 B C5H6O3 C C8H16O5. Which of the following contains the largest number of atoms.
8 atoms of sulphur. So 1g of water means 118 moles 0055 moles. Now if we compare 4g of H 2 has more number of atoms.
A mole is defined as the mass of the substance which consists of an equal quantity of basic units. 1 mole of SO 2 3N A of Atoms. One mole of oxygen molecules contains more atoms than one mole of lead atoms.
100 mole of H2O. 1 mole of each of these substances contains Avogadro number 6022 E23 of atoms. There are 3 atoms in the molecule.
A mole of copper atoms has more atoms than a mole of lead atoms. In addition it was determined that the sample contains 14830 x 1023 hydrogen atoms and 12966 x 1023 oxygen atoms. 8g of SO 2 38 N A of atoms.
Then the bigger amount of oxygen is in the 00553 g of SO3 where there is a bigger number of moles. Mass of Li 1 g. Which one of the following contains largest number of atoms -.
And since Fe iron has the largest of moles slightly more than 2 it also has the largest number of atoms. As 1 mole contains number of Li atoms. Which of the following samples contains the largest number of atoms.
Which has maximum number of molecules. Total number of atoms 3 x 0055 x 6022 x 10 23 1003 x 10 23 atoms. All of these contain the same number of atoms.
1 mol water b. Which of the following 495 g samples contain the fewest number of atoms. 30 moles of NH3C.
1 mol phosphorus trichloride c. 40 moles of ceHNO3 d. 60 moles of ceHClO e.
See the answer See the answer done loading. Now 1 mole of molecule contains atoms. 10 mol of water H 2 O b.
Thus 112 g Fe has the largest number of atoms among the three samples. A 591 g unknown sample analyzed by elemental analysis revealed a composition of 3751 C. A 4g of H2.
50 moles of HCl. Then you divide the amount of grams of each compound by.

Which Of The Following Contains Maximum Number Of Atoms

Calculate The No Of Atoms Of Au In 1 Gm Of Au No Of Molecule Of H2o In A Drop Of H2o Weighting 0 05 Gm Atomic Mass Of Au Is 197

Lewis Structures Chemistry Lessons Chemistry Classroom Teaching Chemistry

Which Has Maximum Number Of Atoms

Which One Of The Following Will Have Largest Number Of Atoms

Which Of The Following Contains Largest Number Of Atoms

Which Of The Following Contains The Greatest Number Of Atoms Scholr

Which Of The Following Contains Largest Number Of Atoms

Which Of The Following Has Maximum Number Of Atoms Youtube
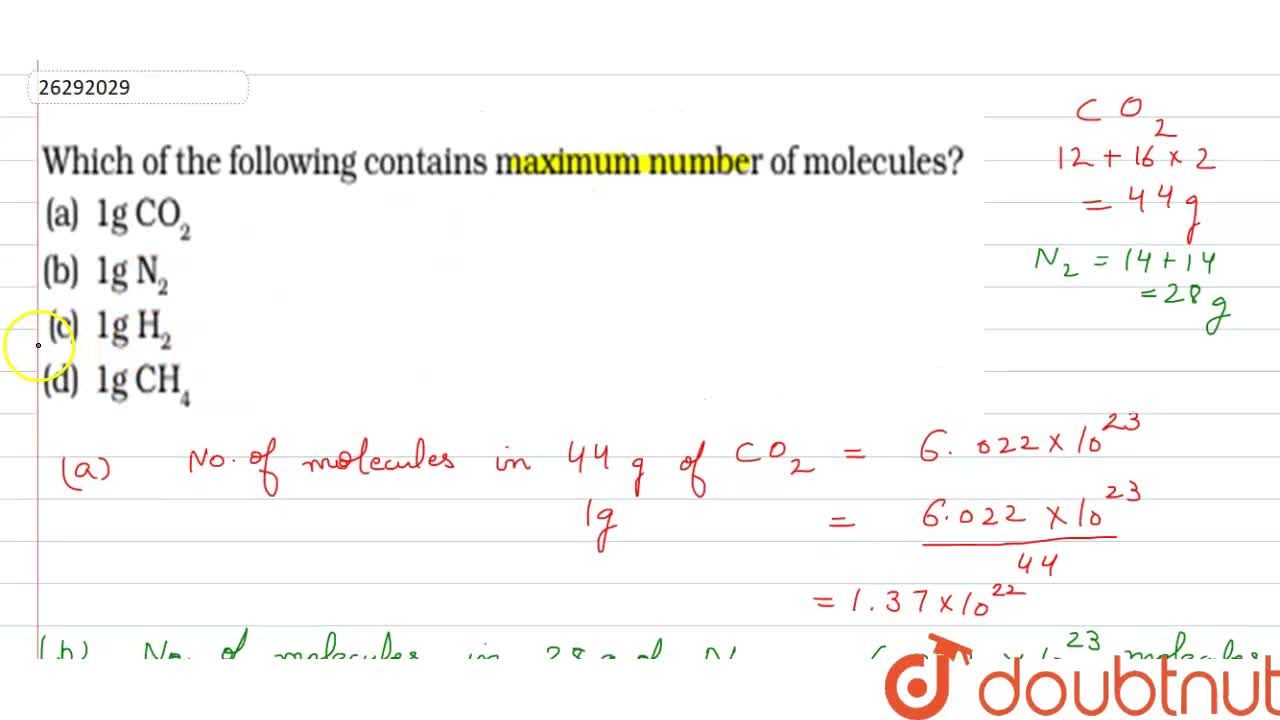
Which Of The Following Contains Maximum Number Of Molecules

2 3 Calculating Atomic Masses Problems Chemistry Libretexts

Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy

Which Of The Following Contains Largest Number Of Atoms

Pin By Jass Manak On Neet 1 Bullet Journal Paper Sheet Music

Science Revision Chemistry Notes Science Revision Science Notes
How Many Atoms Are Present In 28 G Of Nitrogen Quora

Calculating The Number Of Atoms In A Sample Youtube

Which Of The Following Contains Greater Number Of Oxygen Atoms A 1 Gm Of O B 1 Gm Of O2 C 1 Gm Of O3 D All Have Same Number Of Atoms
Comments
Post a Comment